As per WHO, vaccine hesitancy is one of the top ten threats for global health safety. The survey shows that the main reason to the vaccine hesitancy is the lack of confidence on the safety parameters and concerns about adverse events.
To bust this mis notion cloud, let’s understand the science that goes behind vaccine research, testing, safety, and quality check.
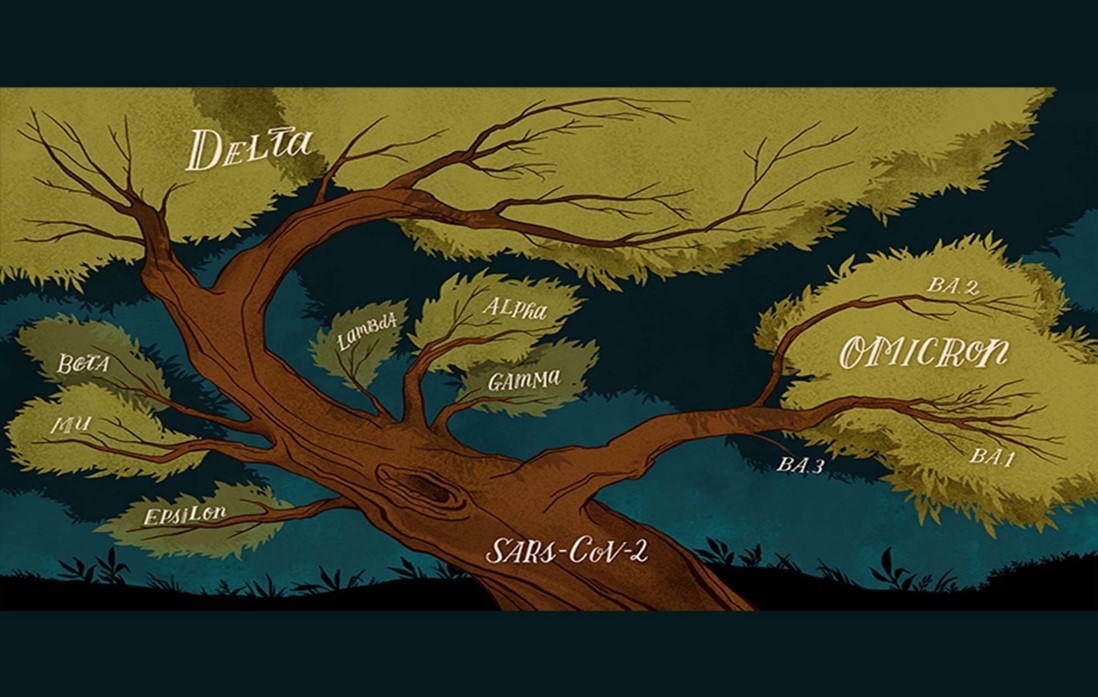
Vaccine Journey
The team of scientist and medical experts from across the globe work together to create a vaccine which takes years of extensive research before it gets available to the community.
- Exploratory – Scientists explores ways to modify virus for safety or build up a molecule that resembles to a part of the virus. This development starts by detecting a target or specific protein that induce the immune reaction. Vaccine is being created which is similar to the target that induce antibody formation against that virus.
- Preclinical Trials – The created molecule or vaccine is tested in animals (in-vitro and in-vivo) to understand vaccine safety and immunological response or toxicity.
- Clinical trials – Post animal studies, vaccine is then tested on human beings in different phases as below:
- Phase 1 – Small group of people receives the vaccine (10-12 group of individuals)
- Phase 2 – Hundreds of segmented people with specific age and characteristic health for which vaccine is intended are administered vaccines
- Phase 3 – Thousands of people are tested with vaccine for its safety and efficacy
Many vaccines progress to phase 4 which checks the performance and study the long-term risks and benefits in real life scenarios.
- FDA Review and Approval
US FDA’s Center for Biologics Evaluation and Research is responsible for vaccine regulation.
Following steps need to be followed to get the approvals:
- Filing an Investigational New Drug application
- Pre-licensure vaccine clinical trials
- Inspection of manufacturing facility
- Presentation of findings to FDA’s Vaccines and Related Biological Products Advisory Committee
- Usability testing of product labeling
Post compliance to all above requirement, FDA continues to keep a watch on production, inspects facility periodically to ensure the safety and efficacy. FDA demands the reports of each lot of vaccines on potency, safety, and purity.
Tracking and Reporting of Adverse events
The Vaccine Adverse Event Reporting System is a national platform that is used to track the safety of US licensed vaccines. This platform can be used by vaccine manufacturers, health care professionals, vaccine recipients or parents to report any significant clinical adverse events which occur post vaccination.
In India, Adverse Events Following Immunization (AEFI) surveillance program is used to monitor suspected adverse events post vaccination.
The efforts that are put in from long years surely make vaccines very safe to use and protects you against life threatening illness.
So, to sum up:
“Don’t doubt the safety and quality, instead vaccinate yourself on priority”